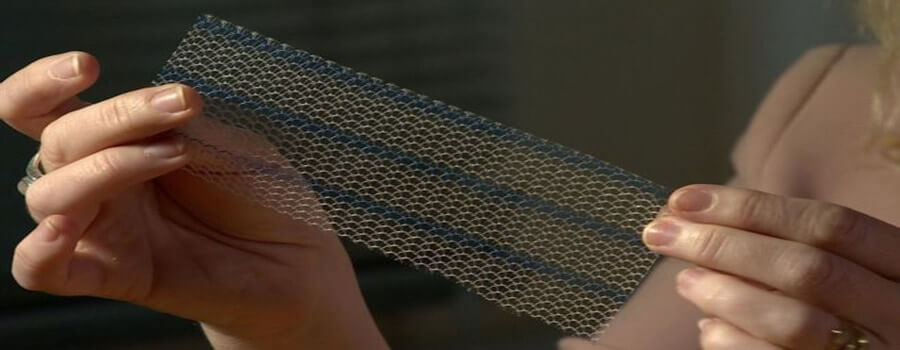
What are vaginal mesh implants?
The implants are medical devices used by surgeons to treat pelvic organ prolapse and incontinence in women, conditions that can commonly occur after childbirth.
Some women with incontinence receive treatment using tension free vaginal tape, although adverse side effects are not thought to be as common.
The mesh, usually made from synthetic polypropylene, is intended to repair damaged or weakened tissue.
An NHS audit was launched earlier this year to understand how many women may be affected by the devices in cases where they deformed after being implanted – in some cases cutting the vagina walls and causing life-threatening infections.
England’s most senior doctor has been told by the government to urgently respond to new data that shows how many women have had vaginal mesh surgery.
Since 2008 27,016 have had the implant – which is used to treat incontinence and prolapse – and 211 women have had it removed.
But there are arguments that the latest figures arent accurate as they dont include data on procedures done privately. It is estimated more than 100,000 UK women have had a mesh fitted. Most of them suffer no ill effects, but some women have reported severe and constant abdominal and vaginal pain following the surgery, and some have been told that they can no longer have sexual intercourse.
Other women have experienced infections and bleeding, while many have said their original incontinence symptoms have not been improved by the surgery.
Some women who experienced problems said they were not aware the implants were permanent.
Are mesh implants the only option?
Non-surgical treatments, including physiotherapy, are routinely offered to women suffering from a prolapsed bladder and/or incontinence. However, in more serious cases traditional surgery – which doesn’t use implants – can be necessary.
However, such surgery has a 20-30% failure rate, which is why many women were offered the mesh implants as an alternative.
Campaigners are calling for the use of the mesh to be suspended, and manufacturers are facing legal action in the UK and overseas.